Technical Tip: Engineering successful analytical methods using HILIC as an alternative retention mechanism
Overview: This technical tip will outline hydrophilic interaction chromatography (or hydrophilic interaction liquid chromatography, HILIC) as an alternative retention mechanism that is particularly effective for LC-MS. HILIC uses high levels of organic solvent to elute the polar compounds, which helps to improve their ionization efficiency during LC-MS.
Modern drug candidates present many challenges chromatographically, be them detection or resolution from a co-eluting impurity. One of the most common difficulties faced is the retention and resolution of polar compounds that are not amenable to typical C18 reversed-phase conditions.
Many traditional reversed-phase HPLC stationary phases do not provide adequate retention for many of the ever-increasing number of polar compounds analyzed by HPLC. These compounds are often either unretained in the void volume of the column, retained so poorly that their peak shape is compromised, or co-elute with other poorly retained peaks. Early-eluting peaks also elute in an ion-suppression zone that is problematic for people using LC-MS instruments.
Tip: HILIC works by retaining polar compounds into a water-rich layer that is formed at the surface of the silica particle as a result of a polar stationary phase (Fig. 1)
Acetonitrile (MeCN) is the weak solvent during HILIC and ammonium buffer the strong solvent. Ammonium salts are critical as a counter ion as it facilitates the formation of the bilayer critical to separation demonstrated in Figure 1.
A polar-rich stationary phase draws a water-rich layer to its surface, which promotes the retention of polar compounds into the stationary phase. Polar compounds are eluted as you increase the amount of water in the mobile portion of the column. This disrupts the retention of polar analytes along the polar stationary-phase to induce elution.
It is important that you fully equilibrate your HILIC column before use to ensure that this water-rich layer has had the appropriate amount of time to form. If this has not occurred, then you will not see consistent retention of your compound or robustness of your method.
We mentioned the use of ammonium buffers, and there are several other factors within a HILIC method that are also critical for its success.
The first is selectivity, for which the correct column is key. As with reversed-phase columns, there are many different stationary phases for HILIC chromatography and they each offer different selectivity profiles and benefits. We would recommend you always endeavor to screen 3 phases of different chemistries to ascertain which offers the best selectivity for your application.
The types of HILIC stationary phases you might consider include:
- underivatized silica such as Kinetex HILIC
- diol phases like Luna HILIC
- NH2 phases
- ionic/zwitterionic chemistries
It is important with mobile phase to use MeCN as the organic component. Protic solvents such as methanol will disrupt this bilayer formation discussed earlier. The amount of organic is also important since if it is too high of a percentage in the initial gradient starting point, it will complicate and prolong the equilibration of the bilayer. The ideal initial method conditions should be 90% organic.
Gradient profile should be 90% MeCN – 50% wherever possible. This ensures that the bilayer is fully equilibrated at the start of the gradient. If conditions greater than 50% buffer are required to elute the compound, then it is likely the method is not best suited to HILIC, and a polar C18 type of stationary phase might be a better option.
You will need to re-equilibrate the bilayer after each gradient run. The higher the buffer percentage at the end of the gradient, the longer this re-equilibration process will take. Incomplete re-equilibration of the buffer will lead to poor reproducibility and a lack of robustness to the method.
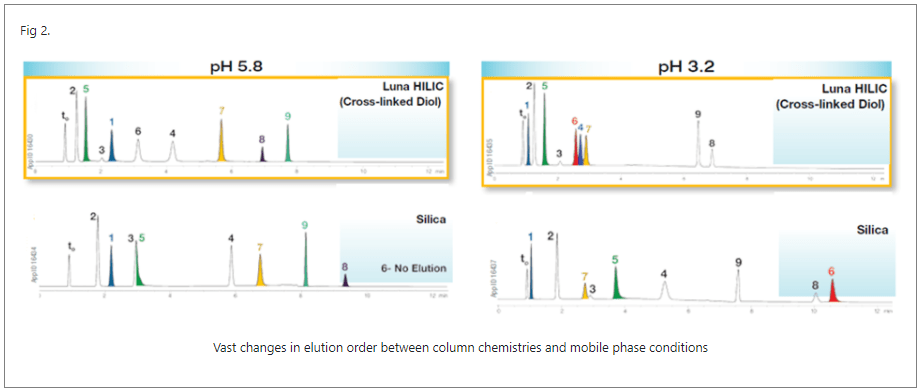
The final parameter you should consider carefully is pH. At least 2 different pH’s should be screened, and we would usually suggest pH 3.2 using ammonium formate and 5.8 using ammonium acetate. Different pH’s can lead to significantly different selectivities (Fig. 2).
HILIC is a remarkably useful tool to advance the retention of polar compounds. The method can be a challenging way to build robustness, but with careful mobile phase choices and method parameters, it can lead to successful separations for taxing compounds.
The information for this article was pulled from the Phenomenex Technical Note – “Engineering successful analytical methods using HILIC as an alternative retention mechanism.”
Have a question or need assistance?
Connect with Phenomenex’s team of Technical Specialists who are available 24/7 around the world through online chat. Chat Now offers a free and quick way to get the answers and assistance you need to succeed in your work. Click the link to start chatting today >> www.phenomenex.com/chat.